Delivery System Development Services
Effective delivery remains a critical challenge in rare liver disease therapies, constrained by complex hepatic physiology and disease-specific barriers. Our integrated platform combines rational design with empirical validation to develop tailored delivery systems addressing hepatocyte-specific targeting, disease microenvironment adaptation, therapeutic payload compatibility, and clinical translation readiness. We focus on optimizing delivery efficiency and therapeutic precision to accelerate the transition from preclinical research to clinical application.
Overview
Background & Significance
Drug delivery systems play a pivotal role in therapeutic development for rare liver diseases, where precise hepatic targeting is essential to maximize efficacy while minimizing systemic toxicity. The liver's unique physiology creates complex challenges for achieving consistent and durable therapeutic effects. Advances in nanotechnology, gene therapy, and biomaterial engineering have expanded the toolkit for overcoming these hurdles, enabling tailored solutions that address both biological and clinical constraints.
Key Delivery Challenges
Effective therapeutic delivery in rare liver diseases faces multifaceted obstacles rooted in the liver's unique physiology and disease pathology. Physiological barriers include fenestrated endothelial structures that restrict particle access, fibrotic tissue impeding diffusion, and altered hemodynamics in cirrhotic microenvironments. Concurrently, therapeutic demands require precise cell-type targeting, stabilization of nucleic acid payloads (siRNA/mRNA), and sustained release mechanisms for chronic conditions-all while maintaining biocompatibility and minimizing off-target effects.
Technology Evolution
The field has advanced through iterative innovations addressing these challenges. Early milestones like GalNAc-siRNA conjugates (2012) enabled hepatocyte-specific RNAi delivery, while AAV8 vectors (2016) set clinical benchmarks for liver gene transfer. Subsequent breakthroughs, including lipid nanoparticle (LNP) optimization (2019) for mRNA vaccines and engineered capsids (2022) enhancing human hepatocyte tropism, reflect a shift toward modular platforms. Current strategies prioritize balancing targeting precision, payload versatility, and scalable production to bridge preclinical efficacy with clinical translation.
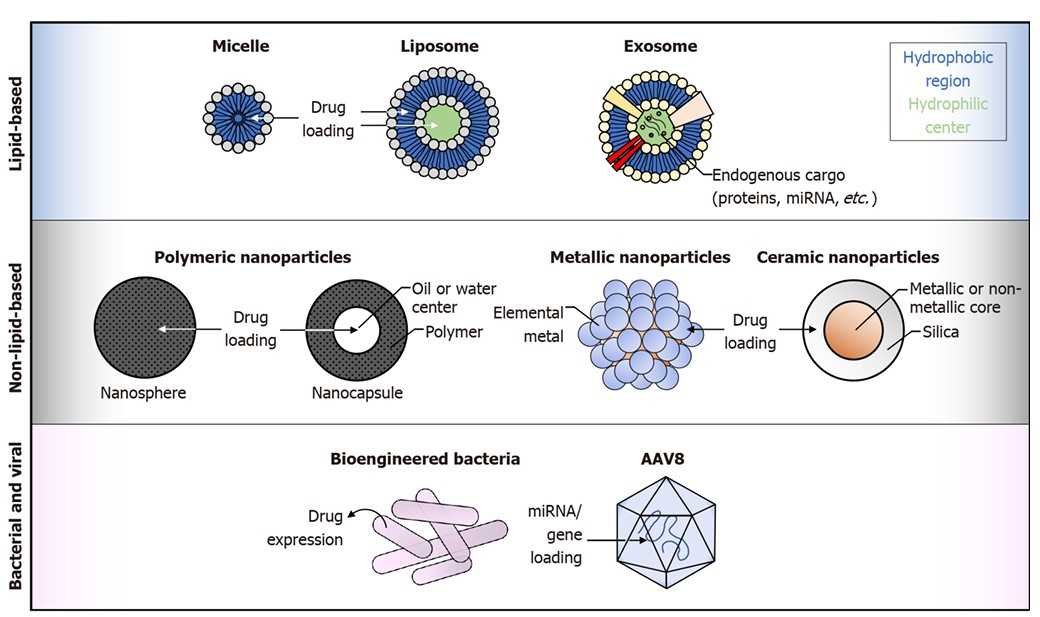 Fig1. Graphical representation of targeted drug delivery platforms. (Warner, et al., 2022)
Fig1. Graphical representation of targeted drug delivery platforms. (Warner, et al., 2022)Our Services
Protheragen's drug delivery system development services cover the entire process from basic research to preclinical evaluation, aiming to provide comprehensive support for the development of drugs for rare liver diseases.
Liver-Targeted Nanocarriers
We engineer lipid- and polymer-based nanocarriers optimized for hepatic delivery, focusing on precise drug loading, controlled release kinetics, and in vivo targeting efficiency validation in liver disease models.
RNAi Delivery Optimization
Our RNAi delivery platforms address siRNA/shRNA stabilization and hepatocyte-specific delivery, with end-to-end evaluation of silencing efficacy, biodistribution, and durability in preclinical studies.
We systematically compare AAV serotypes for liver tropism, providing vector design, production, and in vitro/in vivo transduction efficiency profiling to identify optimal candidates for gene therapy applications.
Hydrogel-Based Sustained Delivery
Hydrogel systems are tailored for prolonged drug release in hepatic microenvironments, including biomaterial optimization, drug release profiling, and biocompatibility testing in disease-relevant models.
Conjugated Therapeutics Development
We design and validate antibody- or polymer-drug conjugates with liver-targeting ligands, ensuring payload stability, targeted biodistribution, and therapeutic safety in preclinical evaluations.
Why Choose Us?
- Integrated Technology Platform
Our end-to-end capabilities span nanocarriers, RNAi platforms, viral vectors, hydrogel-based systems, and conjugated therapeutics, supporting development from early discovery to preclinical stages.
- Tailored Solutions
We design delivery systems customized to therapeutic payload type, target indications, and development phase requirements.
- Expert Scientific Team
A multidisciplinary team with deep expertise in nanotechnology, gene therapy, and pharmacology provides strategic guidance from concept to preclinical validation.
- Reliable Data Output
FAQs?
-
Q: What delivery system options are available for rare liver disease therapies?
A: Our platform includes liver-targeted nanocarriers, RNAi delivery systems, AAV vectors, hydrogel-based sustained-release platforms, and conjugated therapeutics, tailored to address diverse therapeutic requirements.
-
Q: How is the optimal delivery platform selected for a specific therapy?
A: We use a systematic evaluation framework analyzing payload properties (size, stability), dosing regimen (acute/chronic), disease stage (e.g., fibrosis severity), and target cell specificity to match the most effective delivery strategy.
-
Q: What is the typical timeline for custom delivery system development?
A: Development timelines range from 3 to 6 months, depending on payload complexity, required preclinical validation, and formulation optimization steps.
-
Q: Do you provide in vivo delivery efficiency testing?
A: Yes, we conduct rigorous in vivo studies using disease-relevant models to assess targeting accuracy, biodistribution, and therapeutic efficacy of developed systems.
-
Q: Which core technologies underpin your delivery system development?
A: Our work integrates advanced nanotechnology, RNAi optimization, viral vector engineering, and biocompatible material science to ensure precise, scalable, and clinically translatable solutions.
Reference
- Warner JB.; et al. Liver-specific drug delivery platforms: Applications for the treatment of alcohol-associated liver disease. World J Gastroenterol. 2022;28(36):5280-5299.
All of our services and products are intended for preclinical research use only and cannot be used to diagnose, treat or manage patients.
