Pharmacokinetics Services
Protheragen offers specialized pharmacokinetics services designed to support preclinical research for rare neurodegenerative disorders. Our expertise and advanced methodologies ensure robust, actionable data to streamline drug development and reduce risks.
The Role of Pharmacokinetics in Rare Neurodegenerative Diseases
- Understanding Drug Behavior
Pharmacokinetics (PK) studies analyze how therapeutic compounds are absorbed, distributed, metabolized, and excreted (ADME) in the body. For diseases like ALS, Huntington's, and spinal muscular atrophy, these insights are critical to ensuring drugs reach target tissues at effective concentrations for sufficient durations. PK data guides dosing strategies, minimizes toxicity risks, and improves the likelihood of therapeutic success.
- Optimizing Drug Development
PK analysis is central to refining drug candidates for rare neurodegenerative diseases. By evaluating ADME properties early in development, researchers can prioritize compounds with favorable pharmacokinetic profiles—those that balance efficacy, safety, and bioavailability. This approach reduces costly late-stage failures and accelerates the selection of viable candidates for clinical trials.
- Improving Patient Outcomes
Integrating PK data with clinical research enhances treatment predictability and safety. For rare diseases with limited patient populations, PK-driven insights help tailor therapies to individual needs, maximize therapeutic benefits, and minimize adverse effects. This precision ultimately leads to better clinical outcomes and quality of life for patients.
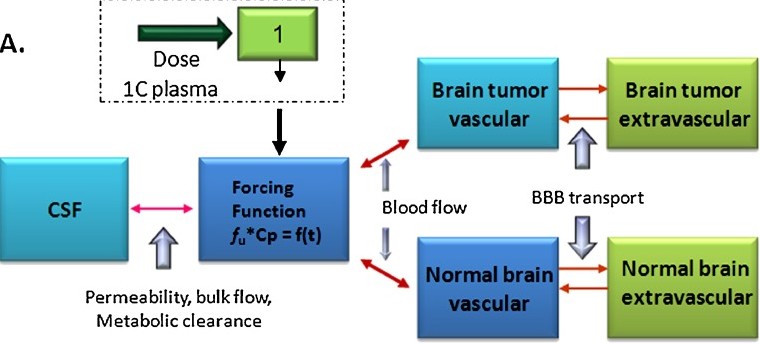 Fig1. Schematic presentation of the hybrid PBPK model of TMZ in brain. (Zhou, et al., 2011)
Fig1. Schematic presentation of the hybrid PBPK model of TMZ in brain. (Zhou, et al., 2011)Our Services
Protheragen delivers tailored pharmacokinetics (PK) services to support preclinical research for rare neurodegenerative diseases. Our end-to-end solutions span early-stage ADME profiling to advanced PK modeling, addressing the unique challenges of rare disease drug development. Combining technical expertise with innovative tools, we provide actionable data to accelerate therapeutic discovery and clinical translation.
Core Services
ADME Profiling
We evaluate how drug candidates are absorbed, distributed, metabolized, and excreted in preclinical models. Key assessments include:
- Absorption: Measuring how compounds enter systemic circulation.
- Distribution: Tracking drug movement to target tissues and organs.
- Metabolism: Identifying metabolic pathways and potential interactions.
- Excretion: Analyzing elimination routes and rates.
Preclinical PK Modeling & Simulation
We predict human drug behavior using preclinical data through:
- PK Modeling: Building mathematical frameworks to map drug concentration over time.
- Dose Simulation: Forecasting optimal dosing regimens for efficacy and safety.
- Data Interpretation: Translating PK results into actionable development strategies.
Biomarker-Driven PK Studies
We integrate biomarkers to enhance PK insights:
- Biomarker Validation: Identifying markers linked to drug exposure or response.
- Concentration-Response Analysis: Aligning dosing with therapeutic effects.
Additional Offerings
- In Vitro ADME Assays: Testing metabolic stability, transporter interactions, and enzyme inhibition.
- In Vivo PK Studies: Evaluating drug behavior in disease-relevant animal models.
- Toxicokinetics: Linking drug exposure to toxicity profiles for safety assessment.
Protheragen equips researchers with precise PK data to optimize drug candidates for rare neurodegenerative diseases. Our ADME profiling, modeling, and biomarker integration services reduce development risks and enhance clinical predictability. By prioritizing rigor and adaptability, we help teams advance therapies with confidence, from preclinical studies to human trials.
Why Choose Us?
- Advanced Tools: We use the latest analytical instruments and computational methods to deliver precise, reliable pharmacokinetics (PK) data for informed decision-making.
- Experienced Team: Our team has hands-on expertise in PK and ADME studies, offering custom strategies and guidance tailored to your project's goals.
- Full-Spectrum Support: From early absorption studies to complex PK modeling, we cover every phase of preclinical drug development to keep your research on track.
- Quality Assurance: Rigorous quality checks and clear reporting ensure data integrity, while adaptable workflows align with evolving project needs.
FAQs
-
Q: What ADME studies do you perform?
A: We handle absorption, distribution, metabolism, and excretion studies, customizing each step to match your compound's unique characteristics.
-
Q: How do you model PK data?
A: We apply computational tools and mathematical models to predict how drugs behave in humans, helping refine dosing for better efficacy and safety.
-
Q: Can biomarkers be part of PK studies?
A: Yes. We track markers linked to drug exposure or response to fine-tune dosing and identify patient subgroups likely to benefit.
-
Q: Do you customize PK services?
A: Absolutely. Whether you need in vitro assays or in vivo studies, we design workflows to fit your specific research questions.
Reference
- Zhou Q, Gallo JM. The pharmacokinetic/pharmacodynamic pipeline: translating anticancer drug pharmacology to the clinic. AAPS J. 2011;13(1):111-120.
All of our services and products are intended for preclinical research use only and cannot be used to diagnose, treat or manage patients.
