
Primary eosinophilic gastrointestinal disorders (EGID) are immune-mediated chronic morbidities that affect mostly children and young adults, resulting in marked disease burden and low quality of life. Protheragen is the foremost service provider in research on uncommon gastrointestinal disorders such as EGID. We provide an all-in-one solution for preclinical drug development and discovery, tailored to shorten your timeline for developing novel therapy options.
Primary eosinophilic gastrointestinal disorders (EGID) consist of a group of diseases with eosinophilic inflammation of particular areas of the bowel in the absence of secondary pathology like infection or reaction to medication.
Recently, based on the location of the inflammation, EGIDs have been classified as eosinophilic esophagitis (EoE) and non-EoE EGIDs. EoE has an estimated incidence of one in two thousand persons; however, it is considered a major contributor to upper gastrointestinal morbidity. Available information regarding the epidemiology of non-EoE EGIDs indicates that they are less common, with a prevalence rate between 3/100,000 and 8/100,000.
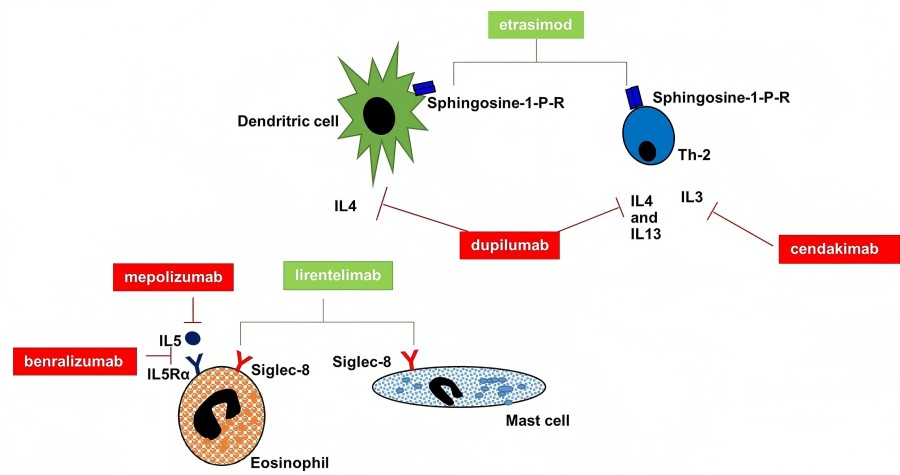
Fig.1 Key molecular targets of monoclonal antibodies in EGID. (Rossi, C. M., et al., 2022)
Both atopy and autoimmunity are linked to EGIDs. Activated eosinophils are pathognomonic of EGID, and their infiltration often causes organ dysfunction. It appears that there is a combination of genetic predisposal combined with environmental risk factors and intestinal dysbiosis which leads these multifactorial diseases known as EGIDs to develop, and triggers the development of T-helper type 2 (Th2) inflammation and epithelial barrier dysfunction.
 Fig.2 Framework for consensus nomenclature in EGIDs. (Dellon, E. S., et al., 2022)
Fig.2 Framework for consensus nomenclature in EGIDs. (Dellon, E. S., et al., 2022)| Drug Names | Mechanism of Action | Targets | NCT Number | Research Phase |
| Benralizumab | Hinder the attachment of IL-5 to its receptor and subsequent heterodimerization of the α and βc subunits. | IL-5Rα | NCT05251909 | Phase III |
| CC-93538 | This is a humanized, particular, recombinant monoclonal antibody for the IL-13 molecule. | IL-13 | NCT05214768 | Phase III |
| AK002 | An anti-Siglec-8 antibody removes tissue eosinophils and improves dysphagia symptoms in individuals. | Siglec-8 | NCT04322604 | Phase III |
Disclaimer: Protheragen focuses on providing preclinical research services. This table is for information exchange purposes only. This table is not a treatment plan recommendation. For guidance on treatment options, please visit a regular hospital.
At Protheragen, our integrated services include the entire scope of preclinical work. We not only propel the innovation of new therapeutic targets and validate drug candidates but also conduct advanced diagnostic studies to discover critical biomarkers and illuminate disease processes. This allows for the creation of effective therapeutic approaches for rare gastrointestinal diseases. Our disease model development systems recapitulate the pathobiological features of EGID, thus serving as an adequate platform for mechanistic studies and therapeutic evaluations.

The impact of animal models on the progress of EGID research is undeniable. These allow researchers to study the immunological and molecular facets associated with the recruitment, activation, and tissue remodeling of eosinophils. With advances in technology, our company provides bespoke animal model development services to target specific areas of research.

Transgenic models that overexpress cytokines lead to increased eosinophil production and recruitment, simulating the inflammatory response observed in EGID.
Optional models:

Specific sensitization and antigen challenges lead to gastrointestinal eosinophilia and inflammation similar to the manifestations of EGID.
Optional models:
Protheragen incorporates specialized preclinical services that assess a candidate's pharmacokinetics and safety to aid in minimizing risks and facilitate easier transitions into human trials. Our unique combination of profound scientific knowledge and modern technology makes us the most reliable partner in altering the terrain of rare gastrointestinal illness research. For inquiries about our services, please reach out to us for further details and pricing information.
References
All of our services and products are intended for preclinical research use only and cannot be used to diagnose, treat or manage patients.