- Home
- Solutions
- By Services
- Ophthalmic Disease Therapeutics Development Services
- Antibiotic Development Service

Antibiotics are a group of antimicrobial compounds that either prevent the multiplication of bacteria or destroy them. The advancement in medicine marked during the early twentieth century with the discovery of antibiotics drastically changed the medical field by saving millions by treating bacterial infections. Protheragen is committed to advancing the field of ophthalmic antibiotic development through innovative solutions and comprehensive services.
Antibiotics, including all other medications in ophthalmology, are essential for treating any ocular bacterial infection. Some of the ophthalmic diseases treated with antibiotics are conjunctivitis, keratitis, blepharitis, and endophthalmitis. Most of these infections can be caused by several bacterial pathogens like Staphylococcus aureus, Pseudomonas aeruginosa, and Streptococcus pneumoniae. Anterior segment infections are best treated with traditional formulations of antibiotics such as eye drops and ointments. However, these medications also have problems regarding some ocular barriers like the tear film and nasolacrimal drainage, as well as the blood-ocular barriers which decrease their bioavailability.
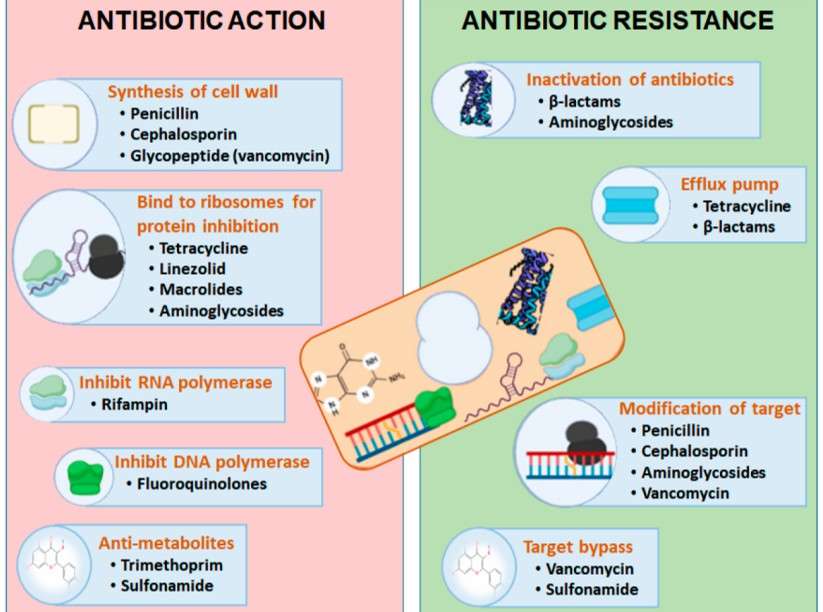 Fig.1 Differentiating the antibiotic action and antibiotic resistance through different aspects within a bacterial cell. (Muteeb G., et al., 2023)
Fig.1 Differentiating the antibiotic action and antibiotic resistance through different aspects within a bacterial cell. (Muteeb G., et al., 2023)Because of the misappropriation and overprescribing of antibiotics, previously treatable infections can now be caused by resistant bacterial strains. Infections in the eye are an example of such infections that are difficult to manage. Such resistance not only complicates the management of ophthalmic infections but also forces the creation of new antibiotics with unique modes of action. The development of novel antibiotics is a multifaceted undertaking requiring vast sums of funding and effort, along with the politics associated with economic and equally as strict governmental orders. In fighting resistant strains of bacteria, while trying to safeguard the susceptible ocular surface, newly developed antibiotics need to be guaranteed as effective and proper.
Overcoming the constraints associated with conventional antibiotic formulations calls for the use of sophisticated delivery systems. The ocular bioavailability of the drug and its residence time on the surface of the eye is achieved through use of liposomes, in situ gelling systems, and nanoparticles. Nanoparticles have the potential to entrap antibiotics, allowing aimed release and release of the drug at the targeted tissues. Liposomes have similar properties, enhanced by better drug retention and lesser drug diffusion into circulation. Drugs with longer duration of action can be administered using in situ gelling systems, which change from liquid to gellable semi-solid form on contact with the tissues of the eye.
Table 1. Antibiotic therapeutics for ophthalmic disease.
| Antibiotics | Targets | Indications | Stages |
| Ciprofloxacin | Bacterial DNA gyrase and topoisomerase IV | Bacterial conjunctivitis, corneal ulcers | Approved |
| Levofloxacin | Bacterial DNA gyrase and topoisomerase IV | Bacterial conjunctivitis, corneal ulcers | Approved |
| Moxifloxacin | Bacterial DNA gyrase and topoisomerase IV | Bacterial conjunctivitis, corneal ulcers | Approved |
| Gentamicin | 30S ribosomal subunit | Corneal ulcers, endophthalmitis | Approved |
| Azithromycin | 50S ribosomal subunit | Chlamydial conjunctivitis | Approved |
| Bacitracin | Bacterial cell wall synthesis | Styes, blepharitis | Approved |
| Doxycycline | Bacterial protein synthesis | Chlamydial conjunctivitis | Approved |
| Sulfamethoxazole | Dihydropterate synthase | Dacryocystitis, preseptal cellulitis | Approved |
Disclaimer: Protheragen focuses on providing preclinical research service. This table is for information exchange purposes only. This table is not a treatment plan recommendation. For guidance on treatment options, please visit a regular hospital.
Protheragen offers customized antibiotic development programs tailored to meet the specific needs of each client. Our comprehensive services encompass the entire spectrum of antibiotic discovery and development, from target identification to preclinical studies.

Target Identification and Validation
By analyzing bacterial genomes, we identify essential genes and proteins that are critical for bacterial survival. Validation of these targets is achieved through genetic and biochemical approaches, such as gene knockouts or inhibition studies, to confirm their role in bacterial growth and pathogenesis.

High-Throughput Screening and Hit Identification
Protheragen employs high-throughput screening (HTS) techniques to rapidly identify compounds with antibacterial activity from large chemical libraries. These screens can be designed to target specific bacterial pathways or to detect broad-spectrum antibacterial activity.

Lead Optimization
Lead optimization involves the chemical modification of hit compounds to improve their potency, selectivity, and pharmacokinetic properties. Protheragen's medicinal chemists use structure-activity relationship (SAR) studies to guide the design and synthesis of analogs with enhanced antibacterial activity.

Preclinical Development
Preclinical development includes in vitro and in vivo studies to evaluate the efficacy, safety, and pharmacokinetics of lead compounds, ensuring that they meet the stringent criteria required for human trials.
Addressing the growing concern with antibiotic resistance makes the creation of ophthalmic antibiotics imperative. Protheragen applies its full range of services to the discovery and development of new antibiotics and remains dedicated to their timely advancement. If you are interested in our services, please feel free to contact us.
References
All of our services and products are intended for preclinical research use only and cannot be used to diagnose, treat or manage patients.