Anal canal cancers represent a small fraction of gastrointestinal malignancies, specifically about 2.5% in North America. Protheragen provides full range of diagnostics and therapeutics development services specialized to the particular needs of anal adenocarcinoma.
Overview of Anal Adenocarcinoma
Anal adenocarcinoma is a rare form of cancer affecting the anal canal and accounts for approximately 5% to 10% of all anal cancers. In contrast to the more prevalent type of anal squamous cell carcinoma, anal adenocarcinoma develops from glandular tissues of the anal canal as well as mucosal tissues from the transitional zone. This type of cancer is frequently seen with chronic inflammatory conditions like Crohn's disease which may cause fistulas around the anus leading to cancer. Due to the low incidence of anal adenocarcinoma, diagnosing and treating it alongside any associated research becomes difficult, requiring a team with varied disciplines for proper management.
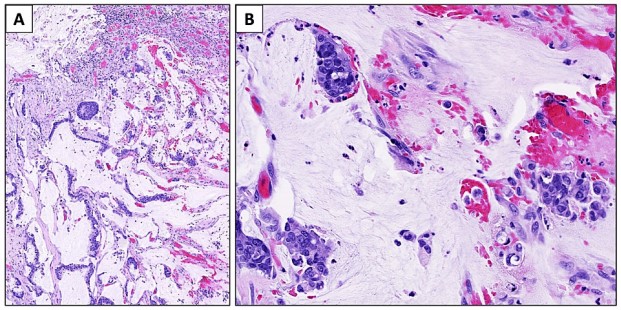
Fig.1 A case study of histopathological analysis of anal adenocarcinoma. (Komornik F. N.,
et al., 2022)
Pathogenesis of Anal Adenocarcinoma
The development of anal adenocarcinoma is due to an interplay of inherited, inflammatory, and environmental factors. Chronic inflammation, such as that which occurs with Crohn's disease, is one of the more important driving factors along with exacerbated mucosal hyperproliferation and dysplasia. Certain genetic alterations in oncogenes and tumor suppressor genes such as KRAS, BRAF and NRAS have been revealed, although they are not universally present. Some tumors also show the presence of microsatellite instability (MSI) and loss of DNA mismatch repair (MMR) proteins, which alters some aspects of tumor behavior and therapeutic response. Smoking is also a known risk factor which further adds to the complexity of the disease's pathogenesis.
Diagnostics Development for Anal Adenocarcinoma
Histopathological Evaluation
For the diagnosis of anal adenocarcinoma, a definitive diagnosis requires histopathological examination. In the case of adenocarcinomas, the biopsy is scrutinized for tumor grade, subtype (e.g. mucinous type), and microsatellite status. Immunohistochemistry enables the evaluation of a number of prognostically important markers such as MLH1 and PMS2, as well as MSH2 and MSH6, which allow estimation of mismatch repair activity and significantly contribute to prognosis.
Laboratory Tests
The diagnosis and follow-up of anal adenocarcinoma may benefit from the ancillary information provided from laboratory tests, including measurement of the levels of carcinoembryonic antigen (CEA). Though not exclusive to the particular cancer, increased CEA levels could suggest the presence of metastasis or an aggravation of the condition.
Therapeutics Development for Anal Adenocarcinoma
- Adjuvant Therapy
Cases with advanced tumor stage (cT3/T4) or node-positive disease, as well as other high-risk features, often receive chemotherapy and radiation adjuvant therapy. For the management of anal adenocarcinoma, some chemotherapy regimens like FOLFOX (which includes fluorouracil, leucovorin, and oxaliplatin) are undergoing evaluation, given their proven advantages in colorectal cancer. Additional radiation therapy may be used to target residual disease or high-risk areas.
- Targeted and Immunotherapy
In instances with certain genetic changes, targeted therapies like anti-HER2 agents can be used. The use of immunotherapy, particularly immune checkpoint inhibitors, demonstrates tremendous potential in metastatic anal squamous cell carcinoma and remains an active research area in anal adenocarcinoma. These therapies seek to utilize the body's immune response to attack the cancer, which may be advantageous in advanced or therapy-resistant situations.
Table 1. Therapeutics of anal adenocarcinoma.
| Drug Name |
Target |
Description |
Stage |
| Capecitabine |
Tumor cells |
An oral fluoropyrimidine carbamate that is a prodrug of 5-fluorouracil (5-FU). It can be used in combination with mitomycin and radiation therapy as an alternative to 5-FU. |
Approved |
| Fluorouracil (5-FU) |
Tumor cells |
A commonly used chemotherapeutic agent in combination with mitomycin and radiation therapy for nonmetastatic anal cancer. |
Approved |
| Mitomycin |
Tumor cells |
Often combined with 5-FU and radiation therapy. It has shown improved local control and colostomy-free survival rates in some studies. |
Approved |
| Cisplatin |
Tumor cells |
Used in combination with 5-FU for chemotherapy. Also studied as a substitute for mitomycin in chemoradiation regimens, with varying results. |
Approved |
| Carboplatin |
Tumor cells |
Combined with paclitaxel, it has shown improved overall survival and fewer serious adverse events compared to cisplatin and 5-FU in advanced-stage anal cancer. |
Approved |
| Paclitaxel |
Tumor cells |
Used in combination with carboplatin for advanced-stage anal cancer therapeutic. |
Approved |
Disclaimer: Protheragen focuses on providing preclinical research services. This table is for information exchange purposes only. This table is not a treatment plan recommendation. For guidance on treatment options, please visit a regular hospital.
Our Services
Protheragen's diagnostics and therapeutics development services for anal adenocarcinoma are characterized by a strong emphasis on scientific rigor, innovation, and efficiency, ensuring high-quality data and accelerated timelines.
- Karyotype Analysis Service
- Omics Analysis Service
- Biomarker Development Service
- Artificial Intelligence Service
- Customized Diagnostics Development
- Anticancer Peptide
- Gene Therapy
- Immunotherapy
- Monoclonal Antibody
- Phytotherapy
- Small Molecule Drug
- Therapeutic Cancer Vaccine
- Human Anal Adenocarcinoma Cell Lines
- Canine AGASACA Organoids
- Chemically Induced Models
- Patient-Derived Xenograft (PDX) Models
- Genetically Engineered Mouse Models (GEMM)
Understanding the particular objectives of a client's research project tells us how best to design services for them. From creating new diagnostic assays and analyzing prospective therapeutic agents to devising preclinical study protocols, Protheragen consistently provides customized, fully-integrated, and precise strategic business solutions that are data-driven. If you are interested in our services, please feel free to
contact us.
References
- Komornik, Fillip N., et al. "Anal adenocarcinoma arising from a fistula-in-ano: a case report." Cureus 14.11 (2022).
- Lukovic, Jelena, et al. "Anal adenocarcinoma: a rare malignancy in need of multidisciplinary management." JCO Oncology Practice 16.10 (2020): 635-640.
All of our services and products are intended for preclinical research use only and cannot be used to diagnose, treat or manage patients.



 Fig.1 A case study of histopathological analysis of anal adenocarcinoma. (Komornik F. N., et al., 2022)
Fig.1 A case study of histopathological analysis of anal adenocarcinoma. (Komornik F. N., et al., 2022)