ZFN-based Therapy Development Platform
Zinc finger nucleases (ZFNs) are artificially modified restriction enzymes that can specifically bind and break down DNA and are widely used in disease research. As an integrated CRO, our company has extensive expertise in ZFN-based therapy development, we can provide one-stop personalized project management services according to the specific requirements of customers. We employ a talented team to provide data analysis, detailed results reporting, and discussions to quickly respond to your rare disease research project.
Structure of Zinc Finger Nucleases (ZFNs)
Zinc finger ribonucleases (ZFNs) consist of a DNA recognition domain and a nuclease domain. The DNA recognition domain is composed of a series of Cys2-His2 zinc-finger proteins connected in series, usually 3-4. Each zinc finger protein recognizes and binds a specific triplet base. The nuclease domain consists of the FokI restriction enzyme and is responsible for the catalytic breakdown of DNA. The zinc finger domain can be genetically engineered so that zinc finger nucleases can target specific DNA sequences in complex genomes and achieve the purpose of precisely changing the genomes of higher animals through the repair of endogenous DNA.
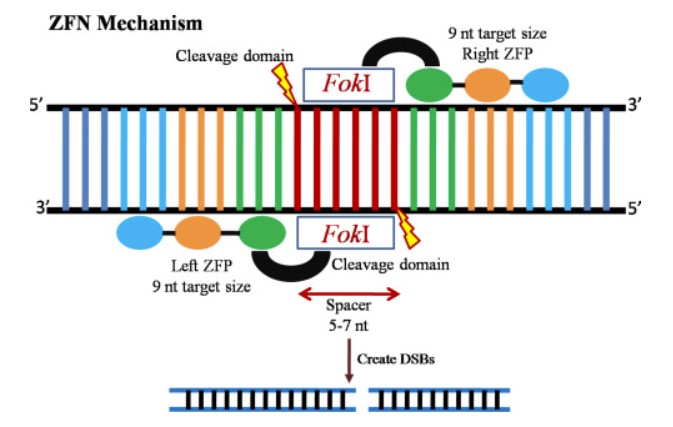
Fig.1 Induction mechanism of zinc finger nuclease (ZFN). (Hillary, V. E., & Ceasar, S. A., 2021)
ZFNs play many advantages in rare disease gene therapy research:
- ZFNs can cause DA double-strand breaks at designated sites, which facilitates targeted genome editing in living cells.
- DNA double-strand breaks stimulate cells to initiate natural DNA repair processes with extremely high specificity.
- Compared with traditional gene recombination methods, ZFN technology improves the efficiency of targeted genome modification by 3-5 orders of magnitude.
- The operation cycle is short, and it only takes 5-8 weeks to obtain permanent, heritable gene deletion, insertion, and modified cell lines.
ZFNs for Rare Diseases
Since 2011, ZFNs have been used to replace a malfunctioning gene called hF9 in the body, and researchers have successfully restored nearly normal blood clotting function in mice suffering from the hemophilia B. The use of ZFNs for genome editing provides more possibilities for the therapeutics of rare diseases.
Table 1 Examples of gene therapy clinical trials for rare disease. (Maldonado, R., et al., 2021)
| Condition | Target | Clinical trial ID | Method | Delivery |
|---|---|---|---|---|
| B-thalassemia | BCL11a | NCT03432364 | ZFN | Non-viral |
| MPS type I | IDUA | NCT02702115 | ZFN + gene suppl | AAV2/6 |
| MPS type II | IDS | NCT03041324 | ZFN + gene suppl | AAV2/6 |
Our Services
Our company uses site-specific zinc finger nuclease to perform gene editing in cells and model tissues, ensuring rapid and high-quality knockout, replacement, and addition of target genes. The zinc finger nuclease-based therapy development services we can provide include but are not limited to:
- DNA-binding Domains Construction
The ability to genetically alter depends primarily on the DNA-binding specificity and affinity of the designed zinc finger protein. With years of project experience and a collaborative technology platform, our company designs and synthetizes zinc finger domains targeting specific DNA sequences containing a series of DNA triads through a 'modular assembly' approach.
- Zinc Finger Nuclease Optimization
Zinc finger nuclease cleavage may lead to the formation of heterodimers, which is likely to cause off-target effects, and ultimately may lead to DNA mismatches and sequence changes, resulting in strong cytotoxicity. Our company provides zinc finger nuclease activity optimization services to reduce off-target effects. Optional methods include but are not limited to:
FokI cleavage domain orientation optimization to improve cleavage activity.
Site-specific nucleases are co-delivered with DNA end-processing enzymes.
Use brief low-temperature incubation conditions to increase nuclease expression levels.
Enrich zinc finger nuclease-modified cells using fluorescent surrogate reporter vectors.
- Zinc Finger Nuclease Delivery
Typically, zinc finger nuclease-encoded genes enter cells via plasmid DNA, viral vectors, or in vitro transcribed mRNA. Our company offers electroporation or cell transfection methods, as well as viral vector-based delivery systems such as integrase-deficient lentiviral vectors (IDLVs) and adeno-associated virus (AAV).
Project Workflow

Our company employs talented and highly trained scientists focused on ZFN-based therapy development for rare disease research. We have the capabilities and resources to provide professional communication and problem-solving support to ensure that we can quickly respond to the changing needs of your rare disease research projects. If you are interested in our services, please feel free to contact us for more details and quotation information of related services.
References
- Hillary, V. E., & Ceasar, S. A. "Genome engineering in insects for the control of vector borne diseases." Progress in Molecular Biology and Translational Science 179 (2021): 197-223.
- Maldonado, R., et al. "Curative gene therapies for rare diseases." Journal of Community Genetics 12 (2021): 267-276.
All of our services and products are intended for preclinical research use only and cannot be used to diagnose, treat or manage patients.
