General Toxicology Evaluation
General toxicity evaluation can enable researchers to understand the toxic mechanism of rare disease drugs on the body and provide a basis for clinical research. Our company is a CRO with many years of experience in toxicology evaluation. Experts with high-level and professional knowledge backgrounds can provide you with comprehensive and high-quality services to meet all your requirements for general toxicity evaluation in rare disease drug and therapy research.
What is General Toxicology Evaluation?
General toxicity evaluation refers to the ability of a drug to produce an overall toxic effect on the experimental animal body at a certain dose, a certain contact time, and a certain contact method. General toxicity evaluation is the basis for safety assessment and is crucial for drug efficacy research. General toxicity evaluation includes acute, sub-acute, sub-chronic, and chronic toxicity evaluation.
Acute Toxicity Evaluation
Acute toxicity evaluation refers to a test involving one or multiple exposures within 24 hours and is the first step in toxicity research. It is required to use rodent or non-rodent animals.
Sub-acute Toxicity Evaluation
Sub-acute toxicity evaluation refers to the short exposure period, usually 3 months, or the short-term exposure to the poison that causes functional and/or structural damage to the body.
Sub-chronic Toxicity Evaluation
Sub-chronic toxicity evaluation refers to the toxic effects that occur when experimental animals are exposed to larger doses of drugs for many consecutive days.
Chronic Toxicity Evaluation
Chronic toxicity evaluation refers to the toxicity of a drug after long-term low-dose effects in the body. The study period is generally greater than 6 months.
How Is General Toxicology Evaluation Performed?
General toxicity evaluation is generally conducted on experimental animals. The species of non-clinical experimental animals are divided into rodents (rats, mice, guinea pigs, rabbits) and non-rodents (dogs, monkeys). Different administration routes and dosages can be set, and toxicity studies can be conducted by observing physiological, hematological, physiological, biochemical, and pathological indicators. In addition, a combination of in vivo and in vitro toxicity evaluation can be used to conduct a more comprehensive evaluation of drug toxicity.
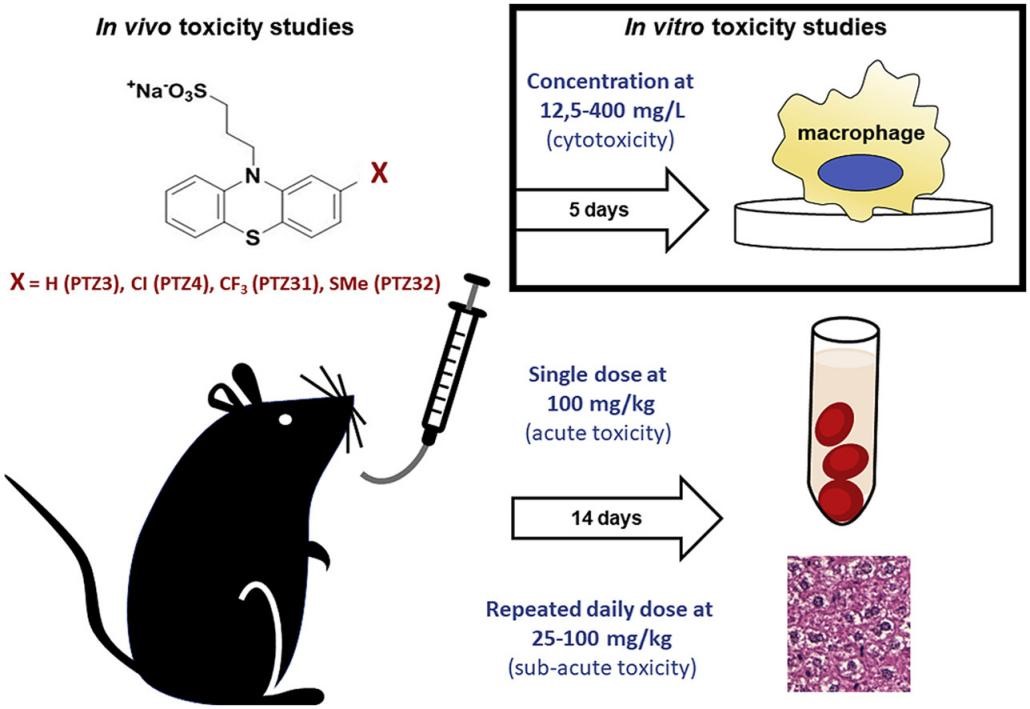 Fig.1 Schematic diagram of general toxicology evaluation. (Salie, S., et al., 2019)
Fig.1 Schematic diagram of general toxicology evaluation. (Salie, S., et al., 2019) Our Services
Our company has an experienced toxicology research team and a complete experimental animal platform to support general toxicology research. Our professional knowledge can provide customers with standard and innovative toxicology research solutions, promptly handle unexpected findings or toxicity issues in the project, and follow up and communicate with you on project progress at any time.
Dose Range Finding
Studies
Dose range finding studies are the first stage of toxicological assessment. Our company has the capabilities and resources to provide professional communication and services to determine doses and provide the basis for later long-term toxicological studies.
Single-dose Toxicity
Evaluation
Single-dose toxicity evaluation can determine the maximum tolerated dose (MTD) and identify potential target organs affected by the compound. Our company has extensive expertise in single-dose toxicity evaluation, and can accelerate your development of rare disease therapy.
Repeated-dose Toxicity
Evaluation
Repeated-dose toxicity evaluation can determine the adverse toxicological effects of repeated daily dosing to a substance within a specific period. Our company can carry out entire repeated dose toxicity evaluation to accelerate your research on the therapeutics of rare diseases.
Project Workflow

Why Choose Us?
Our company offers comprehensive services and technical support covering every stage of rare disease therapy research and development, designed to help our clients address possible barriers and challenges in general toxicology evaluation. If you are interested in our services, please feel free to contact us for more information.
Reference
- Salie, S., et al. "In vitro and in vivo toxicity evaluation of non-neuroleptic phenothiazines, antitubercular drug candidates." Regulatory Toxicology and Pharmacology 109 (2019): 104508.
All of our services and products are intended for preclinical research use only and cannot be used to diagnose, treat or manage patients.
