Development of Gene Therapies for Rare Diseases
More than 80% of rare diseases are single-gene genetic disorders and lack effective treatments. Gene therapy can alter the biology of living cells by modifying or manipulating gene expression, offering the possibility of correcting underlying genetic defects to address the underlying cause of rare diseases. Successful gene therapy may require only a single dose to bring about lifelong improvement without the need for lifelong continuous treatment. In recent years, with the development of genetic diagnostics for rare diseases, advances in vector delivery technologies and biotechnologies such as CRISPR-Cas9, gene therapy has become one of the key research directions for therapeutic drugs for rare diseases.
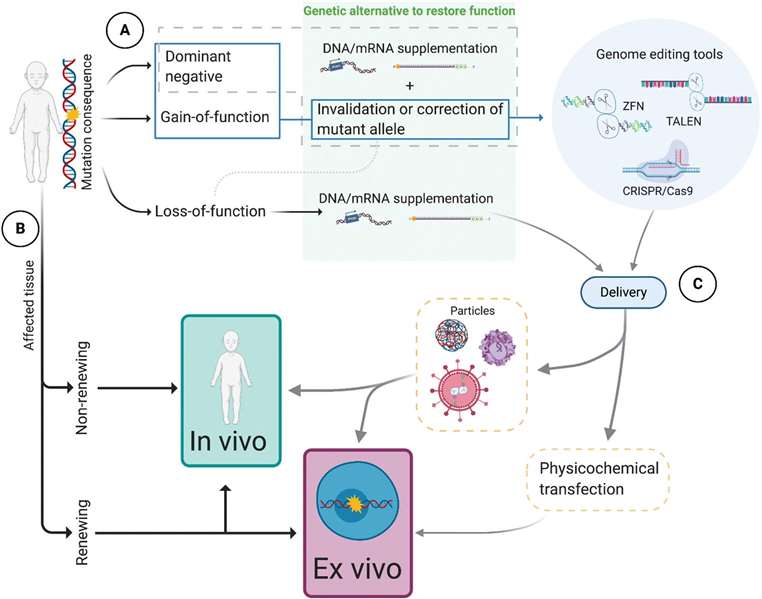
Fig. 1 Flow chart model of biological and technical variables describing gene therapy strategies. (Maldonado R, et al., 2021)
In recent years, our company has made significant advances in gene testing and gene editing technologies, precision medicine, and personalized medicine. This has laid the groundwork to help our clients exploit gene therapy potential in rare diseases. We provide innovative biotechnology companies, research institutions, and pharmaceutical companies with comprehensive gene therapy development services including viral and non-viral vector development, gene editing technology support, and gene therapy safety enhancement to accelerate the development of safer and more effective gene therapies for rare diseases.
Our Services

We provide scientific services and technical support to our clients at every critical step of gene therapy development for rare diseases. Our one-stop gene therapy development solutions include target gene selection and preparation, gene delivery, target cell selection, cell transfection, and exogenous gene expression and detection. The following are the specific services we provide and we adjust the scope of services according to the specific needs of clients.
- Nucleic Acid And Plasmid Analysis
We are committed to providing our clients with automated and highly sensitive analytical methods to fully measure and characterize nucleic acids and plasmids, which are essential for the discovery and manufacture of gene therapies for rare diseases.
Development of Rare Disease Gene Therapy Vectors
- Development of Gene Therapy Viral Vectors
We are committed to providing our customers with comprehensive gene therapy viral vector development services using cutting-edge technology, including the design and construction of suitable viral vectors and the production and purification of viral vectors. - Engineering of Targeted Viral Gene Delivery Vehicles
Our company is dedicated to providing customers with optimization services of viral gene delivery vehicles to generate viral vectors with novel gene delivery capabilities, thus meeting multiple gene delivery needs in the treatment of rare diseases. - Characterization of Gene Therapy Viral Vectors
Our company is committed to providing robust tools for the physical and functional characterization of viral vectors to help customers gain a comprehensive understanding of the physical properties of gene therapy viral vectors. - Development of Non-viral Gene Delivery Vectors
Our company is committed to helping our customers design and develop more novel, highly efficient, and less toxic multifunctional non-viral vectors to further accelerate the progress of gene therapy for rare diseases. - Characterization of Non-viral Gene Delivery Vectors
Our company offers a variety of biophysical techniques as complementary analytical tools to help our customers characterize the properties of various non-viral vectors for gene therapy of rare diseases. - Development of Viral- and Non-viral-Based Hybrid Vectors
Our company is committed to helping customers develop viral and non-viral-based hybrid vector systems using suitable non-immunogenic nanomaterials to achieve minimal side effects, optimal tissue targeting, and enhanced efficacy.
Development of Rare Disease Gene Therapies
- Development of Cell-Based Gene Therapies for Rare Diseases
Our company is committed to making new innovations and breakthroughs in CAR molecular design, transduction methods, and the selection of optimal cell types to develop next-generation CAR-T and gene therapies for the treatment of rare genetic diseases. - Development of RNA Therapies for Rare Diseases
Our company is committed to providing a complete scientific pathway for the development of antisense oligonucleotide (ASO), microRNA (miRNA), small interfering RNA (siRNA), and other therapies aimed at accelerating gene therapy for rare diseases. - Development of Epigenetic Therapies for Rare Diseases
Our company is committed to exploring new epigenetic targets and developing and testing epigenetic drugs with longer half-lives, better bioavailability, and improved safety profiles, opening new avenues for the treatment of rare diseases.
Gene Editing Services for Gene Therapy of Rare Diseases
- TALEN Services in Gene Therapy
Our company is committed to providing our customers with an all-in-one solution for gene editing based on TALEN technology, including experimental design, vector construction, and functional validation, to accelerate their rare disease gene therapy development projects. - Meganuclease Services in Gene Therapy
Our company helps customers develop product candidates through meganuclease-based gene editing platforms aimed at using novel therapeutic modalities such as gene editing to address rare genetic diseases.
How Our Company Addresses Gene Therapy Development Challenges
- Reducing costs and time by enabling more efficient processes through product and service expertise
- Scaling up gene therapy manufacturing capabilities to meet the growing demand for gene therapy
- Providing standardized upstream platform solutions
- On-site technical process development support
Our company offers comprehensive services and technical support covering every stage of gene therapy development, designed to help our clients address possible barriers and challenges in the development of gene therapies for rare diseases. Our extensive experience in providing gene therapy solutions allows us to provide you with customized gene therapy products and services as well as comprehensive assistance that can help you move from gene therapy development to gene therapy commercialization. If you are interested in our services, please contact us for more details.
Reference
- Maldonado, R.; et al. Curative gene therapies for rare diseases. Journal of Community Genetics, 2021, 12: 267-276.
All of our services and products are intended for preclinical research use only and cannot be used to diagnose, treat or manage patients.
